Recently, Associate Professor Li Xiang, Professor Sun Yifei, Associate Professor Sun Ye, all from Beihang’s School of Energy and Power Engineering, along with their collaborators, achieved significant progressin research on liquid organic hydrogen carriers. This research was published in Nature Communications under the title “Combination of nanoparticles with single-metal sites synergistically boosts co-catalyzed formic acid dehydrogenation.”
Hydrogen, recognized for its green, pollution-free qualities and high energy density, is considered a key component for decarbonizing the aviation industry. However, achieving a lightweight, efficient, and safe storage and transportation system for hydrogen fuel on aircraft is essential to making hydrogen-powered aviation a reality. Chemical storage and release of hydrogen have emerged as a straightforward and safe alternative for hydrogen storage and transportation. Currently, precious metal-based catalysts dominate the field of (de)hydrogenation reactions, but due to their high cost, there is a pressing need to develop highly active, selective, and affordable non-precious metal heterogeneous catalysts.
The research team introduced a new class of low-cost cobalt-based catalysts (Co-SAs/NPs@NC) in which highly dispersed single-metal sitesare synergistically combined with small defined nanoparticles allowing efficient formic acid dehydrogenation. The team also revealed the mechanism by which nanoparticles modulate the electronic structure of single atoms, enhancing the adsorption and activation of formic acid molecules, thereby pioneering a new approach for designing single-metal atom catalysts.
The optimal material with atomically dispersed CoN2C2units and encapsulated 7-8 nm nanoparticles achieves an excellent gas yield of 1403.8 mL·g−1·h−1 using propylene carbonate as solvent, with no activity loss after 5 cycles, which is 15 times higher than that of the commercial Pd/C. In situ analytic experiments show that Co-SAs/NPs@NC enhances the adsorption and activation of the key intermediate monodentate HCOO*, thereby facilitating the following C-H bond breaking, compared to related single metal atom and nanoparticle catalysts. Theoretical calculations show that the integration of cobalt nanoparticles elevates the d-band center of the Co single atoms as the active center, which consequently enhances the coupling of the carbonyl O of the HCOO* intermediate to the Co centers, thereby lowering the energy barrier.
In this study, the researchers synthesized a cobalt single-atom and nanoparticle composite catalyst using a simple ZIF pyrolysis method. The optimal catalyst, Co-SAs/NPs@NC-950, demonstrated peak activity in propylene carbonate, with a gas production rate of 1403.8 mL/(g·h), surpassing most reported non-precious metal catalysts and outperforming commercial 5% Pd/C and 5% Pt/C catalysts by 15 and 10 times, respectively (see Figure 1).
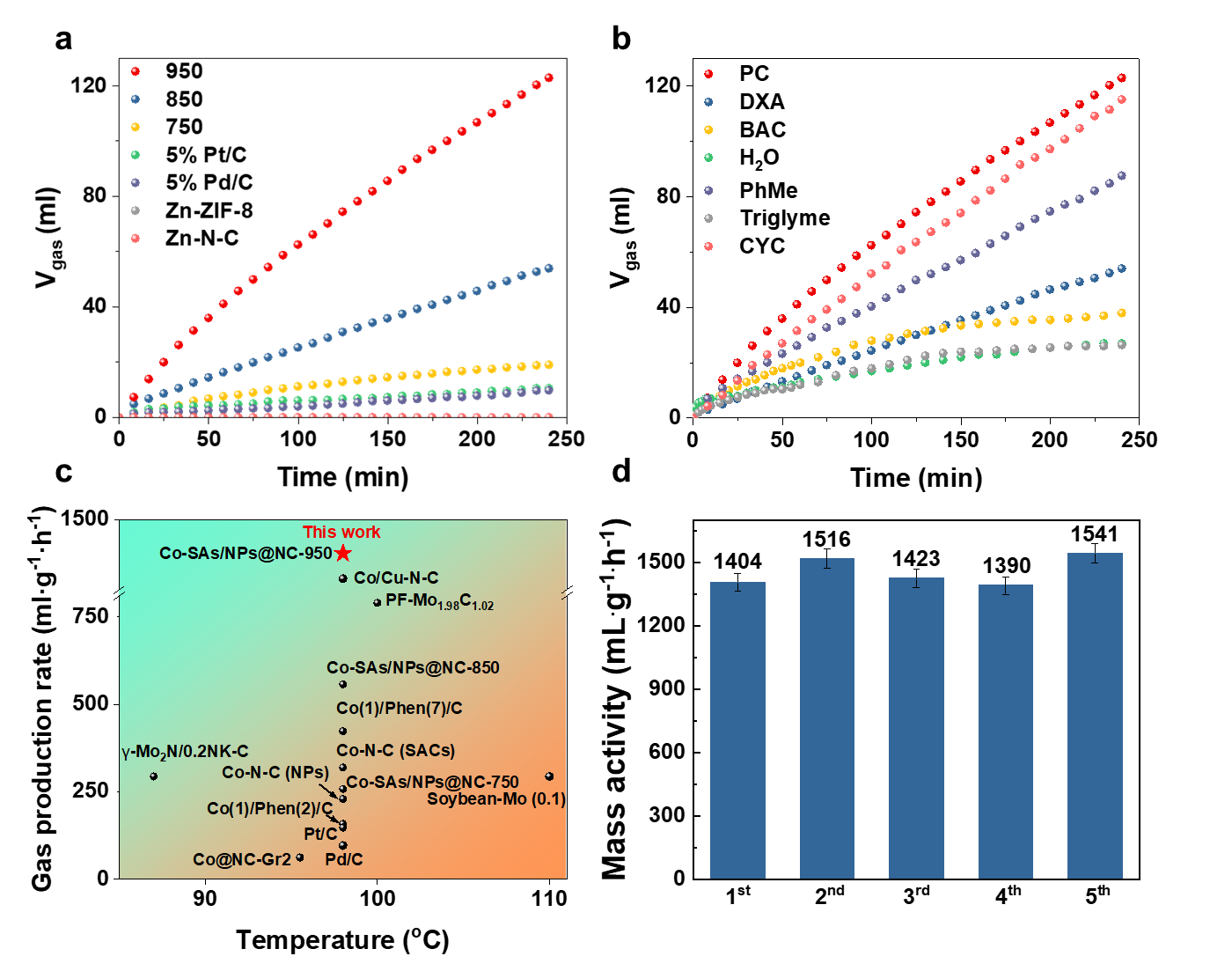
Figure 1: Catalytic performance of Co-SAs/NPs@NC-T
Using in situ infrared spectroscopy and DFT theoretical calculations, the study revealed that the addition of cobalt nanoparticles enhances the adsorption and activation of the key intermediate HCOO*, significantly reducing the rate-limiting C-H bond dissociation energy barrier (0.34 eV) and demonstrating superior formic acid dehydrogenation activity (see Figure 2). This research offers new insights into the development of chemical hydrogen storage and release materials and technologies in the context of hydrogen energy.
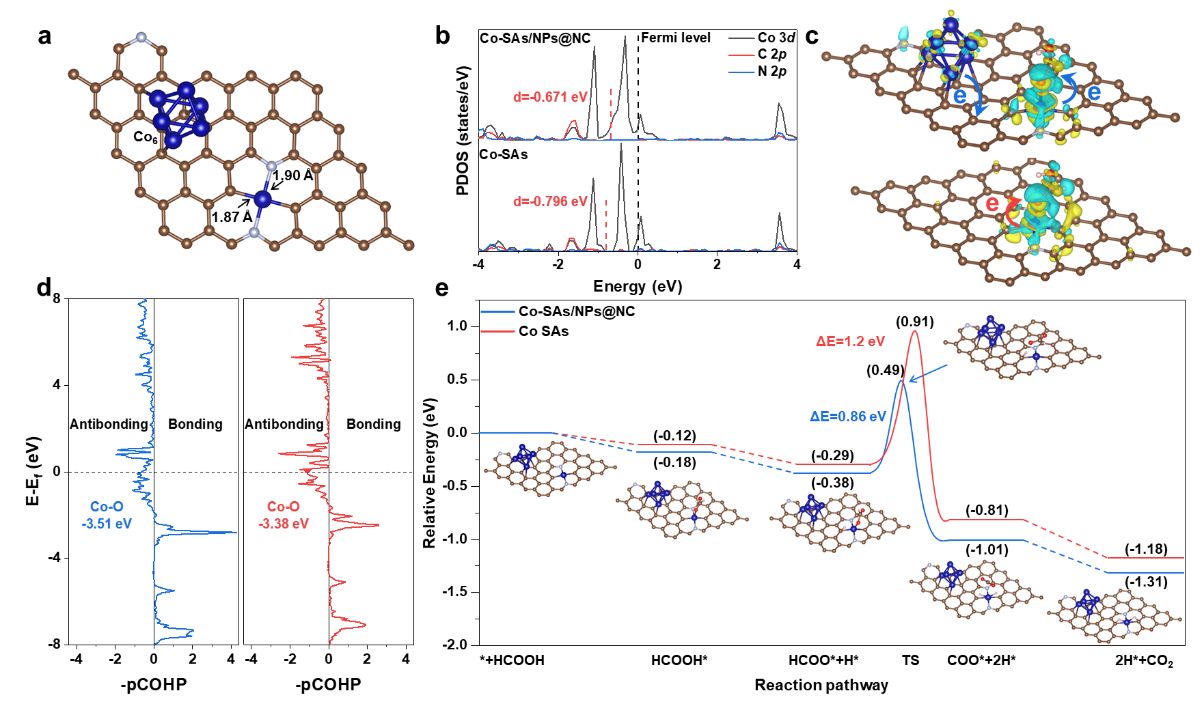
Figure 2: First-principles calculations of Co-SAs/NPs@NC-950
Associate Professor Li Xiang, Professor Sun Yifei, and Associate Professor Sun Ye of Beihang University have long been dedicated to exploring cutting-edge areas in new energy technology development and pollutant control, achieving a series of advancements. Their related research has been published in renowned journals such asNature Communications,Angewandte Chemie International Edition, andEnvironmental Science & Technology.
Beihang PhD student Shi Yanzhe and Professor Luo Bingcheng from China Agricultural University areco-first authors. Beihang Associate Professor Li Xiang, Professor Matthias Beller from the Leibniz Institute for Catalysis in Germany, Beihang Professor Sun Yifei, and Associate Professor Sun Ye are the corresponding authors. This research was supported by the National Key Research and Development Program and the National Natural Science Foundation of China.
This work was financially supported by the National Natural Science Foundation of China and the National Key Research and Development Program.
Original article link: https://doi.org/10.1038/s41467-024-52517-w
Reviewed by: Li Jianwei
Edited by: Jia Aiping
Translated by: Lu Meili

